Electronic Device History Records (eDHR)
Digitised regulated medical device records, replacing paper-based work packs. Automated reporting, improved traceability, and reduced operator workload whilst ensuring full compliance and adoption by staff.
Excel VBAProcess ImprovementComplianceManufacturingAutomationInternal Tools

💥 Impact
Replaced manual paper processes, reduced errors, and improved compliance tracking and audit readiness.
✨ Highlights
Operators quickly adapted to the digital system and appreciated being involved in its development, resulting in high engagement and satisfaction.
📝 Details
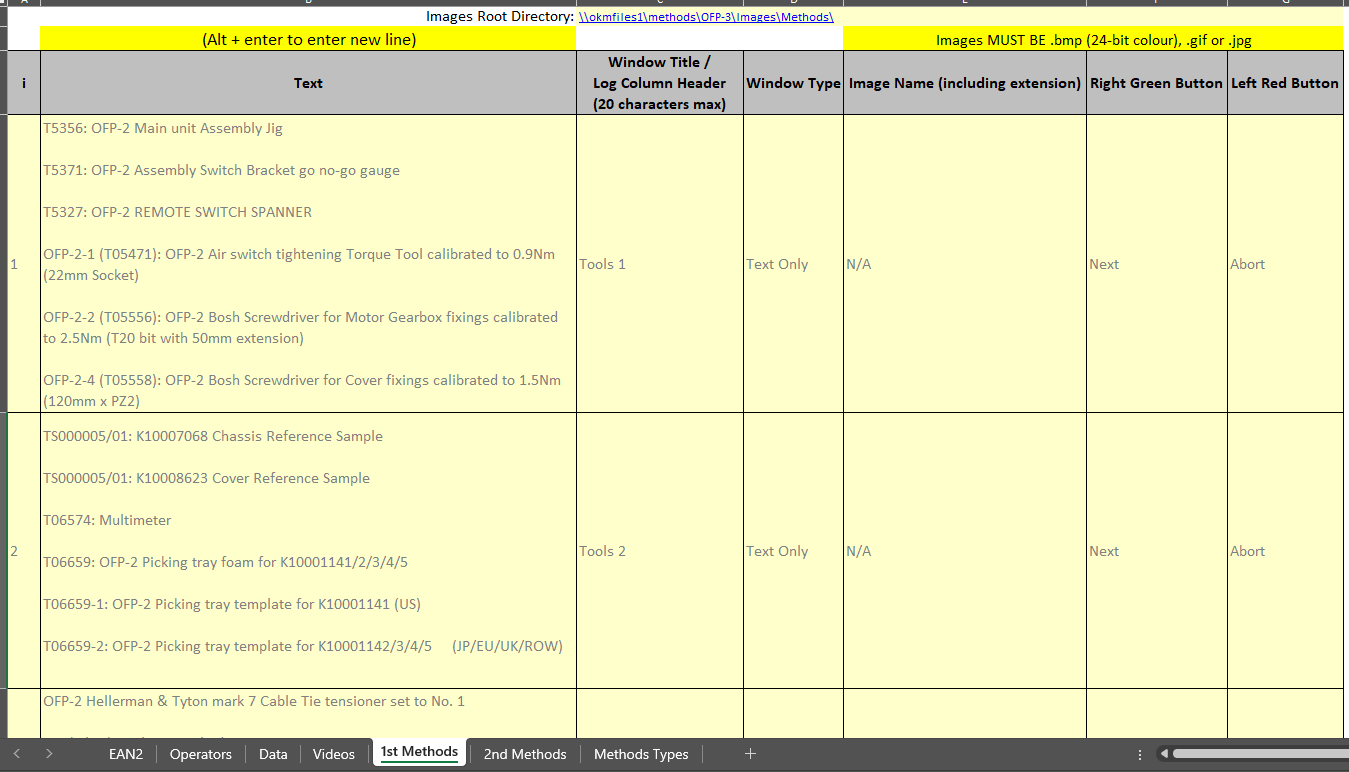
- Digitised Device History Records for medical device builds, replacing paper-based forms.
- Configured validation rules to prevent incomplete or incorrect records.
- Automated audit-ready reporting with full electronic traceability.
- Included multimedia support and randomised button placement to reduce operator fatigue and improve workflow efficiency.
🤝 Contributions
- Designed and developed the digital work pack and eDHR system, with configurable steps, checkboxes, dropdowns, and multimedia support.
- Implemented randomised positions for 'OK' and 'Next' buttons to reduce operator fatigue.
- Worked closely with quality and regulatory teams to ensure compliance and secure internal validation and verification.
- Led stakeholder engagement and operator involvement to refine UX and ensure adoption.
🗓️ Timeline
Initial release within a few months; iterative improvements delivered based on feedback.
🧑🤝🧑 Team
Sole contributor; led all aspects of design, development, and stakeholder coordination.